Elias Barriga Group
Biophysical Mechanisms of Morphogenesis
Research Mission
What synchronises tissue morphogenesis?
A question that inspired research in our group is how collective behaviours are so finely coordinated that cells within tissues move and differentiate with impressively high reproducibility in both time and space. Thus, our long-term goal as a group is to assist the field by training future generations and by producing data sets and tools that will contribute to the conception of a comprehensive view of the mechanisms underlying the robustness of tissue morphogenesis.
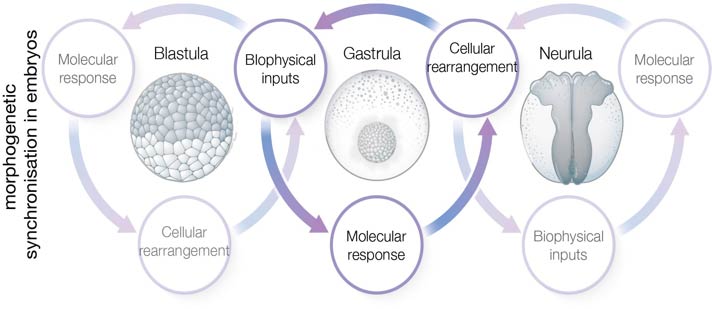
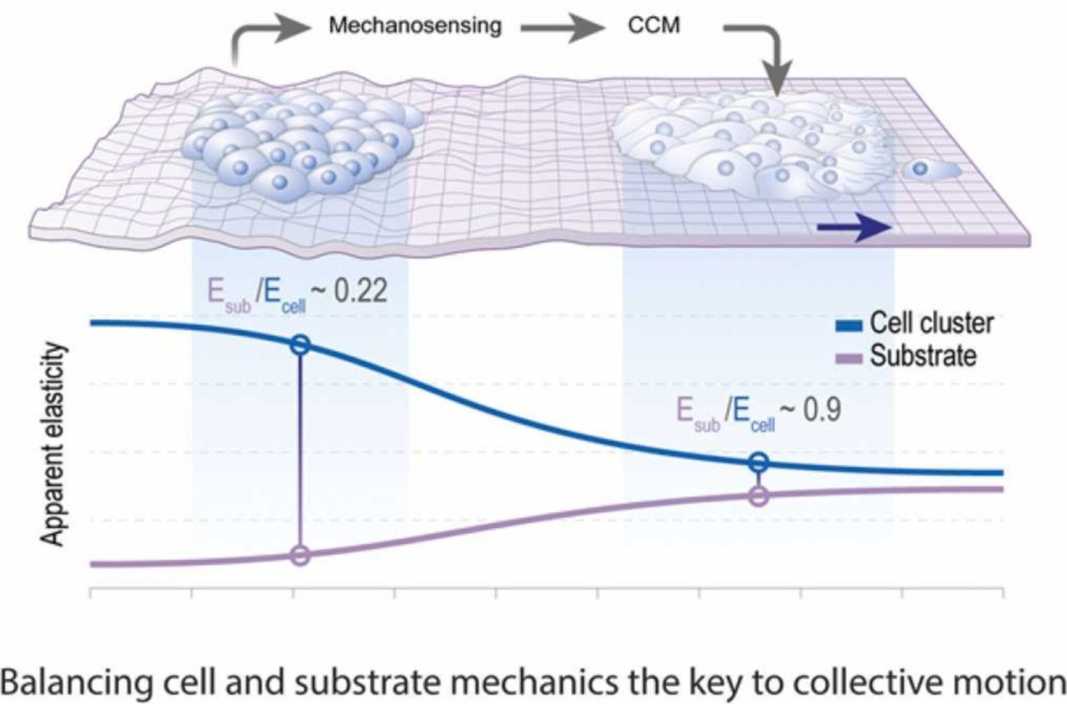
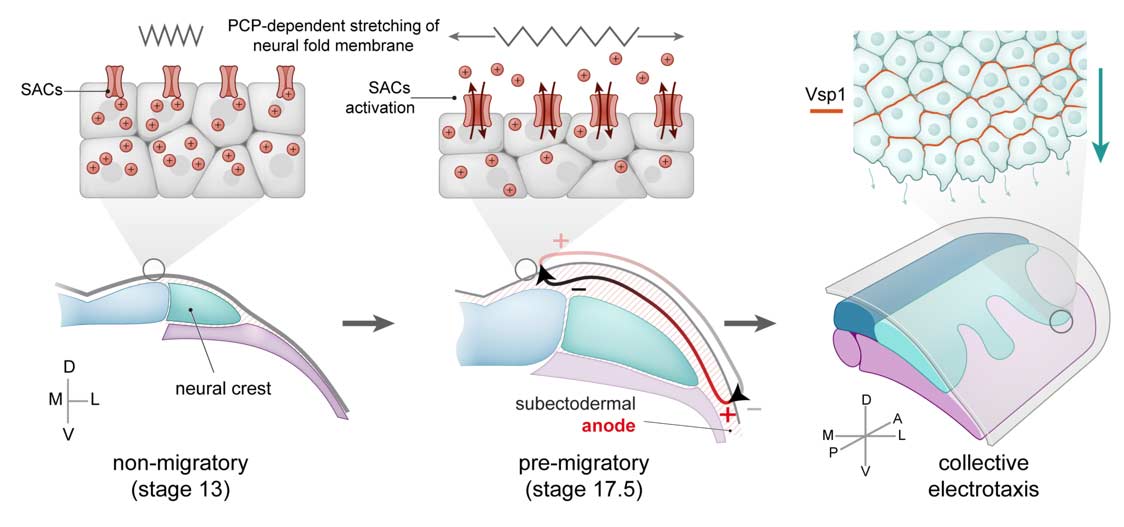
In our multidisciplinary group we are particularly interested on how biophysical quantities (like mechanical or electrical stimuli) can emerge from tissue interplay and how these biophysical stimuli are molecularly integrated into cells to influence collective behaviours such as migration and differentiation, to synchronise morphogenesis (see figure 1). For this we have generated and adapted toolboxes that allow us to measure and modify biophysical properties of living tissues. Using these tools, we are contributing to elucidate the mechano-molecular feedback loops that trigger for the onset of collective movements during embryogenesis (see figure 2). More recently we incorporated tools that enabled us to measure and modify endogenous electric fields to explore the bioelectrical nature of collective cell behaviours in living tissues (see figure 3).
In our group we use Xenopus laevis (frog) and zebrafish as animal systems. To study collective behaviours in embryos we look at cranial neural crest cells as well as hatching gland cells, and to study biophysical control of tissue repair use both frog and fish larval tail regeneration assays. Our cutting-edge toolbox includes in vivo-Atomic Force Microscope, self-referring vibrating probes systems, ionophore probes; glass micro-electrodes, hydrogels, and multiple microfabrication approaches. This, in combination with our molecular tools allow us not only to measure biophysical properties in vivo, but also to modify these properties to quantitatively assess their role in collective cell behaviour during tissue morphogenesis.
References
Figure 1:
Figure 2:
Figure 3: