Inside each cell, individual structures known as organelles perform key functions, but how these organelles contribute to the formation of tissues and organs is unknown. Groundbreaking research from the Campàs group at the Cluster of Excellence Physics of Life of TU Dresden now reveals that the cell’s nucleus controls the stiffness of eye and brain tissues, and even the ordered arrangements of cells in them. These results add a new role for the cell’s nucleus in tissue organization, well beyond its established role in genetic regulation.
Our lives are defined by interactions. Just as people associate with one another, our cells are no different. During development, constant physical interactions between cells give rise to embryonic tissues and organs. Like factories or roads in cities, a myriad of organelles perform tasks inside cells for them to properly function. Because they are confined within cells, organelles were not believed to play a direct role in building organs during embryogenesis. Until now.
The cell’s nucleus is known for processing information in cells, with genes turning on and off depending on the signals received. However, the nucleus is also the largest and stiffest organelle in cells, and could affect the physical structure of the tissue in addition to processing information. Otger Campàs, Professor and Chair of Tissue Dynamics at the Cluster of Excellence Physics of Life of TU Dresden, where he also serves as Managing Director, was particularly fascinated with how the physical properties of the nucleus could play a role in tissue formation. Building on work started during his previous position at the University of California, Santa Barbara, Professor Campàs decided to study the role of nuclei in the formation of the vertebrate eye and brain. “We were measuring tissue stiffness in the zebrafish retina, and realized that it depended on the packing of nuclei. This was totally unexpected because tissue mechanics is believed to depend on cell surface interactions, but not organelles inside cells“. This was a new, unexplored avenue of research that was essential to understand how cells orchestrate embryonic development.
Previous pioneering work by his group had found how forces acting between cells can ‘melt’ the tissue into a fluid state to shape embryos. Now they discovered that nuclei can pack so strongly that they eventually rigidify the tissue. As co-first author Sangwoo Kim states, “By extending the Active Foam Model, we identified a new mode of solid-to-fluid transition, governed by the relative nucleus and cell sizes”.
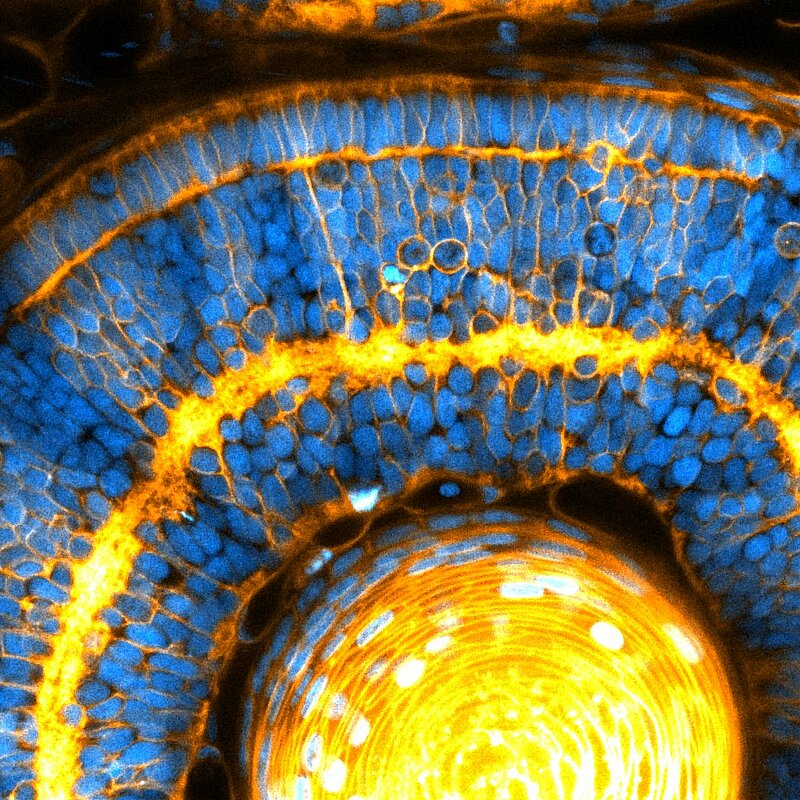
To explore how the size of the cell’s nucleus affects organ formation, the researchers employed zebrafish. These vertebrates are an invaluable model to explore developmental questions, as they are fully transparent during their embryonic stages and mature rapidly, allowing the visualization of organ formation in 3D. “We therefore conducted structural measurements and cell movement quantifications, focusing on the developing retina and brain of zebrafish” highlights co-first author Rana Amini. With these measurements, the authors demonstrated that changes in cell and nuclear sizes during key stages of development ‘jam’ the nuclei into place, as they become tightly surrounded by their neighbours. During this transition, nuclei neatly fit together, like coffee beans in a jar, and this organisation may be important for the eye to function. In our eyes, the packing of cells is very structured, often displaying a crystalline order necessary to process visual cues. In zebrafish it is no different, and the ‘crystalline’ order of cells appears to be a result of the jamming of nuclei as the eye develops.
Beyond the eye, the team also found that brain tissues becomes nuclear jammed, revealing a new role for the nucleus to control the architecture of several neural tissues. This work also highlights a potential role for defects at the nuclear level to cause diseases associated with impaired tissue architecture. With this new piece of the puzzle, we are one step closer to understanding how cells build organs during embryonic development.
Investigators: Sangwoo Kim, Rana Amini, Shuo-Ting Yen, Petr Pospisil, Arthur Boutillon, Ilker Ali Deniz, and Otger Campàs
Funding: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, and the Deutsche Forschungsgemeinschaft (DFG) under Germany's Excellence Strategy, Cluster of Excellence Physics of Life of TU Dresden.
Study: Sangwoo Kim, Rana Amini, Shuo-Ting Yen, Petr Pospisil, Arthur Boutillon, Ilker Ali Deniz, Otger Campàs (2024): A nuclear jamming transition in vertebrate organogenesis. Nature Materials. DOI: 10.1038/s41563-024-01972-3
About the Cluster of Excellence Physics of Life
Physics of Life (PoL) is one of three clusters of excellence at TU Dresden. It focuses on identifying the physical laws underlying the organization of life in molecules, cells, and tissues. In the cluster, scientists from physics, biology, and computer science investigate how active matter in cells and tissues organizes itself into given structures and gives rise to life. PoL is funded by the DFG within the framework of the Excellence Strategy. It is a cooperation between scientists of the TU Dresden and research institutions of the DRESDEN-concept network, such as the Max Planck Institute for Molecular Cell Biology and Genetics (MPI-CBG), the Max Planck Institute for the Physics of Complex Systems (MPI-PKS), the Leibniz Institute of Polymer Research (IPF) and the Helmholtz-Zentrum Dresden-Rossendorf (HZDR).
www.physics-of-life.tu-dresden.de
Contact:
Dr. Kaori Nakashima
Science Communication Officer
Cluster of Excellence Physics of Life
Tel.: +49 351 463-41517
pr.pol@tu-dresden.de