Biomolecular condensates are dynamic assemblies of proteins or nucleic acids, that play key roles in cellular functions (such as gene regulation, division, and nuclear-cytoplasmic shuttling). Both the phase behaviour and material properties of condensates can be changed through alterations in intrinsically disordered protein (IDP) sequences. However, while IDP sequence-specific effects on phase separation have been studied, how exactly these sequences change the material properties of condensates is largely unknown.
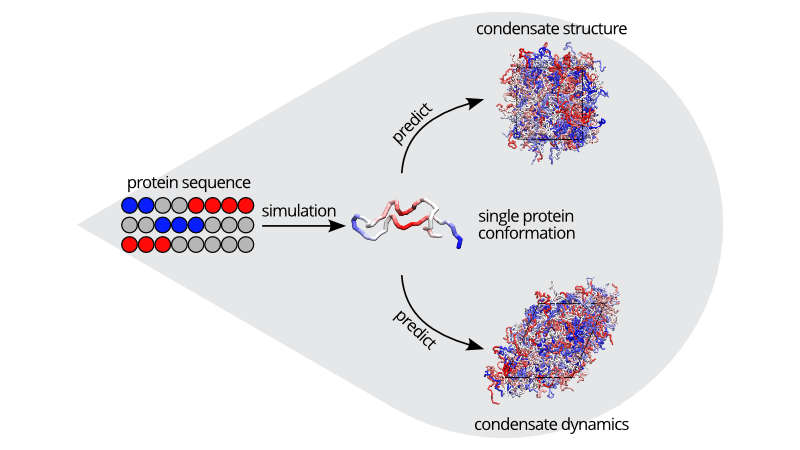
In a recent study published in Nature Communications, Devarajan et al. demonstrate that sequence charge patterning can help modulate the material properties within the dense phase as well as at the interface of condensates, without changing external conditions (such as temperature and salt concentration). The authors utilised a large variety of charge-rich IDP model sequences to develop coarse-grained simulations. These simulations can reveal insights into the dynamical, rheological, and interfacial characteristics of biomolecular condensates at a (semi-)quantitative level.
Material properties of model and natural IDPs were found to respond similarly to charge segregation, despite differences in sequence. The authors also observed strong correlations between single IDP conformations and the resultant material properties of condensates. This phenomenon was explained by analysing molecular interactions, revealing a significant link between the lifetime of noncovalent bonds formed by oppositely charged residues and the material properties of all investigated sequences.
This work demonstrates that short equilibrium simulations of IDP sequences offer a means to characterize their conformations and intra-molecular contact lifetimes, providing valuable insights into the material properties of charge-rich IDP condensates. As single-chain conformations and corresponding condensate material properties were consistent across all respective coarse-grained models, this indicates the universality of these findings.
The molecular insights described here shed light on why specific point mutations in protein sequences can significantly impact the emerging material properties and functions of biomolecular condensates. These data also highlight the utility of single-chain simulations in rapidly exploring varied protein sequences, thus expediting the design and prototyping of (bio)polymers with desired material properties in future.