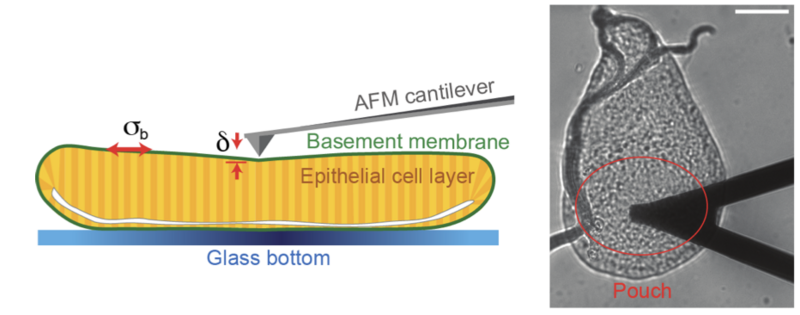
A substantial portion of the animal body is composed of epithelial tissue. During embryogenesis, epithelia folds through complex shape changes to eventually attain their final and functional conformation, giving rise to our organs. This is often dependent on changes in mechanical tension at the apical (facing the external environment) and basal (facing the internal environment) surfaces of cells. How tension is regulated on the apical side of cells has already been established, but basal tension regulation had not yet been validated. The basement membrane, connected to the basal surface of epithelia, has long been suspected of playing a role in this process, but its contribution to tension remained unknown. The lack of direct mechanical measurements of the basement membrane meant this question could not be answered, until now.
Guerra Santillán et al. have recently identified the basement membrane and the actomyosin network (an important component of the cytoskeleton) as key players in generating basal tension. Harnessing atomic force microscopy (AFM), the authors were able to directly measure mechanical tension in Drosophila. Through a combination of AFM and live imaging of the wing disk epithelium, the authors hypothesise that expansile stretching of the basement membrane, and the resulting elastic stress, are key to generate basal tension in epithelial homeostasis. Finally, the authors take one step further, and suggest a model of cellular force to explain how, during homeostasis, intracellular processes can maintain elastic stretching of the basement membrane.
With this new finding, fundamental differences in the mechanical regulation of apical versus basal epithelial tension are now described. This research enhances our understanding of how tissue (and organs) form and maintain shape, which has broader implications for developmental biology, and applicability in fields such as tissue engineering.