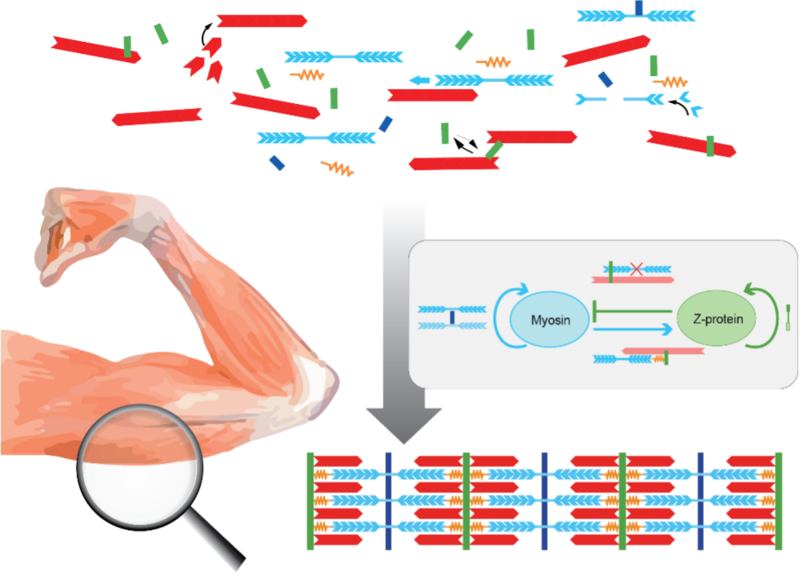
Walking, swimming, jumping, and even heartbeat are powered by long chains of micrometer-sized, contractile units, the sarcomeres. Yet how sarcomeres are assembled during muscle formation is not well understood.
Using new methods to analyze images of developing muscle, Kolley et al. were able to identify which components assemble first, and which follow later. Their data suggests that as the first step, motor proteins named myosin and specific crosslinker proteins form an alternating periodic pattern, while actin filaments, a third key sarcomeric component, are still unpatterned and serve as a scaffold.
Knowing how the different components assemble in time helps to set up mathematical models. In their model, the authors propose that myosin and Z-disc proteins bind and unbind to and from a scaffold of actin filaments, with nonlocal interactions between these spatially extended molecules. Mechanical feedback on so-called catch-bond crosslinkers that bind more tightly when put under tension by molecular myosin motors can additionally contribute to sarcomeric pattern formation.
Thus, by combining experiment and theory, the authors propose a new mechanism that can explain the formation of regular sarcomeres during muscle formation. In addition, their new theory suggests future experiments to further understand how to “build muscle” and eventually understand muscle diseases.
Original publication:
F. Kolley, C. Sidor, B. Dehapiot, F. Schnorrer, B.M. Friedrich: Mechanisms of sarcomere assembly in muscle cells inferred from sequential ordering of myofibril components. PRX Life 2, 013002 – Published 11 January 2024