Gastrulation is one of the most intensely studied biological processes. During gastrulation, animal embryos undergo tissue flows and folding events that transform a single-layered blastula (a hollow sphere of cells) into a complex multi-layered structure known as the gastrula. During this process, many animal embryos need to pull and close a protective layer of cells around the egg. The spreading cells eventually forms a continuous layer that entirely surrounds the embryo and the yolk sac. This spectacular process is called epiboly and its mechanism is not well understood.
During the process of spreading, the cells, that form a continuous, so-called epithelial sheet, are mechanically stressed: first, in order to cover the entire egg, the sheet has to expand and then, once the egg equator is reached, the expanding tissue must shrink again to seal seamlessly at the bottom of the egg. It’s as if one would pull a swimming cap over one’s head like a ski mask. In both the swimming cap and the epithelial sheet this obviously generates mechanical stress that needs to be released. But how does the tissue manage to do this? Scientists from the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, the Institute of Molecular Biology and Biotechnology of the Foundation for Research and Technology Hellas (IMBB-FORTH) in Heraklion, Greece, the Janelia Research Campus at the Howard Hughes Medical Institute in Ashburn, USA, and the Center for Systems Biology Dresden (CSBD) set out to answer this question by looking at the development of the red flour beetle Tribolium castaneum.
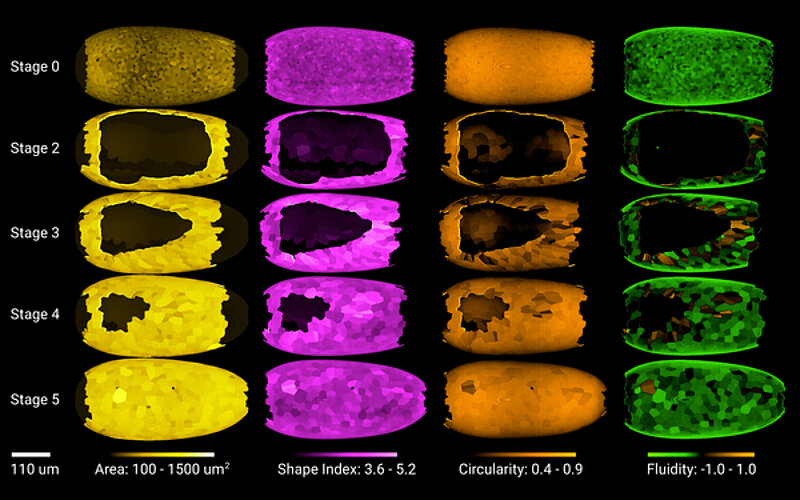
With the help of the cutting-edge technology, available at the MPI-CBG and the CSBD, the researchers found a mechanism that alters the epithelial tissue into a fluid-like state, so it can spread easier over the roughly spherical egg. Akanksha Jain, a recently graduated PhD student in the research lab of Pavel Tomancak at the MPI-CBG, uncovered this mechanism in her PhD project. She explains: “We worked together with the institute’s Light Microscopy Facility and applied advanced image analysis and computational modelling methods to observe and analyze the process of fluidization in the epithelial sheet. To probe the actual tension in the spreading tissue, we used laser cutting.” The researchers described an actomyosin cable at the edge of the epithelial sheet closing around the egg. The cable shrinks and eventually seals the spreading tissue at the bottom of the egg. The amount of actomyosin, a biological substance that provides cells with the ability to contract, is different in different cells at the edge of the spreading sheet. This leads the cells with most actomyosin to shrink faster compared to its neighbors and such cells tend to leave the edge of the closing sheet. In this way, the actomyosin cable contributes to the fluidization of the tissue by tossing cells from the margin and releasing the stress accumulated from the stretching of the sheet around the egg.
Pavel Tomancak, who supervised the study, summarizes: “Since this developmental solution, utilized during epiboly, resembles the mechanism of wound healing, we suggest that this fluidization process could be a conserved mechanism for the closure of epithelial gaps. This realization allows us to use evolutionary reasoning to understand how cells exploit physical mechanisms to close holes in tissues during normal development and after injuries.”
Original publication:
Akanksha Jain, Vladimir Ulman, Arghyadip Mukherjee, Mangal Prakash, Marina B. Cuenca, Lokesh G. Pimpale, Stefan Münster, Robert Haase, Kristen A. Panfilio, Florian Jug, Stephan W. Grill, Pavel Tomancak and Anastasios Pavlopoulos: Regionalized tissue fluidization is required for epithelial gap closure 1 during insect gastrulation. Nature Communications, 5 November 2020. doi: 10.1038/s41467-020-19356-x